Shim/ASU
- “Diamond planets” in the universe could contain the substance in abundance.
- Researchers tested the existence of such planets by subjecting silicon carbide – a mineral thought to exist on carbon-rich planets – to extreme heat and pressure.
- With the addition of water, the mineral was converted into diamonds and silicon, showing that diamond planets are possible.
- Visit Business Insider’s homepage for more stories.
On Earth, diamonds are considered precious partly because they’re fairly rare: The planet’s diamond content is about .001%.
But on other planets in the universe, diamonds may be as common as ordinary rocks.
According to new research published in The Planetary Science Journal, planets with high carbon-to-oxygen ratios – known as carbide exoplanets – could form large quantities of diamonds if those planets also contain water.
The researchers behind the finding, a team from Arizona State University and the University of Chicago, found that under high-heat, high-pressure conditions like those found inside the Earth, certain planets may be making vast quantities of diamonds beneath their surfaces. So much so, in fact that they’re primarily made of the material.
These carbide exoplanets contain far more carbon than Earth. Near their cores, the planets are are also theorized to contain molten silicon carbide, a molecule made up of silicon and carbon.
By simulating the conditions on such a planet in the lab, the researchers confirmed that if heat and pressure get extreme enough — and if a planet has water — silicon carbide can convert to silica and super-compressed carbon crystals, otherwise known as diamonds.

Shim/ASU/Vecteezy
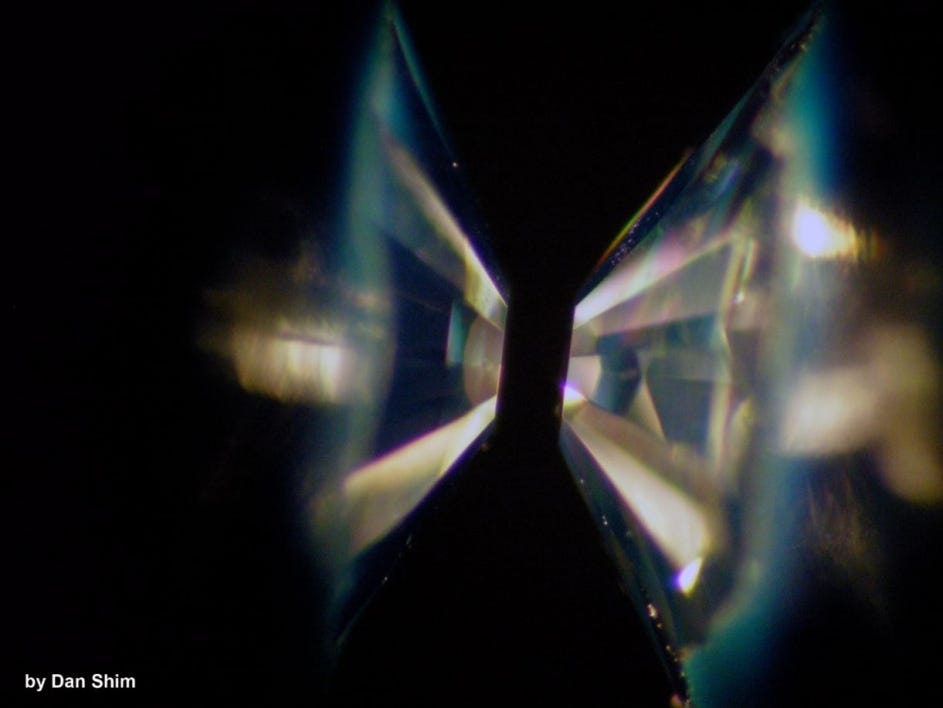
In a lab, the researchers mimicked what this chemical reaction would look like on a carbon-rich planet by first submerging silicon carbide in water. Then they placed samples of the material into a device called a diamond-anvil cell, which consists of two anvil-shaped diamonds that can compress small bits of material using extreme pressure when pressed together. The scientists then superheated the pressurized sample using a laser.
At the end of the process, the sample had indeed converted into diamonds and silica – just as the researchers had predicted. It was confirmation that, yes, it's possible diamond planets exist.
"These exoplanets are unlike anything in our solar system," Harrison Allen-Sutter, the study's lead author and graduate assistant at Arizona State's School of Earth and Space Exploration, said in a press release. That's because carbon-rich planets can only exist near stars with relatively high carbon-to-oxygen ratios, which our sun lacks.
Diamond planets, assuming they exist, would have harsh environments: The researchers theorized that their atmospheres would have to be rich in methane and other gases that are low in oxygen. Plus, the planets would be too hard to be geologically active – a characteristic that keeps temperatures stable.
For these and other reasons, diamond planets aren't likely to harbor life.